




IBDoc

Description of IBDoc
IBDoc® is an in vitro diagnostic immunoassay for the quantitative determination of fecal calprotectin in human stool. It is intended as an aid to the assessment of inflammation of the intestinal mucosa for inflammatory bowel disease (e.g. Crohn’s Disease and Ulcerative Colitis) monitoring.
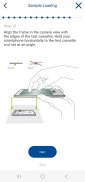
As part of the IBDoc® calprotectin home test, IBDoc® app allows measuring the calprotectin concentration in a stool sample using a smartphone. The app contains an easy to understand tutorial explaining the test procedure step by step from the collection of the stool sample to sample extraction and loading of the pregnancy test-like test cassette. Once the test cassette is prepared, the IBDoc® app controls the mobile device camera to take a picture of the test cassette. The optical signal of the test is analyzed with the help of a specific barcode on the test cassette. The signal is translated into a result that indicates the calprotectin concentration in the stool sample.
The test information is managed on the smartphone itself and at the same time transmitted via a secure and encrypted connection to the IBDoc® Portal. The test results and connected patient information are stored securely on the IBDoc® Portal and can only be accessed by both the patient and his responsible healthcare practitioner.
The IBDoc® app features include:
- Tutorial with schematic drawings and text explaining the test procedure step by step
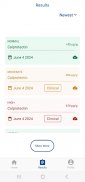
- A list of all test results performed by the patient
- Help menu with a tutorial video and support contact
---------------------------------------------------
IMPORTANT NOTICE
---------------------------------------------------
Please be aware that BDoc® app only works with an IBDoc® Account and an IBDoc® test kit. This Account has to be set up by a healthcare practitioner. Please contact your IBD doctor or IBD clinic for additional information.
Please read the Instruction for Use included in the IBDoc® test kit carefully before performing the first test.
The IBDoc® app has been validated for specific smartphone models to ensure accurate results, please consult the list of validated smartphones here: www.ibdoc.net/support
A data connection is required. Depending on your carrier additional costs might arise.
--------------------------------------------------
For IBDoc® app (BI-IBDOCAND and BI-IBDOCIOS) and IBDoc® Portal (BI-IBDOCPOR) software device:
We, BÜHLMANN Laboratories AG, declare under sole responsibility that the device specified above meets the provision of the IVD Regulation (EU) 2017/746 for in vitro diagnostic medical devices, the Canadian Medical Devices Regulations SOR/98-282, and is in conformity with other relevant Union legislations, common specifications (CS) and other normative documents.
CE-marked product: CE0123
Health Canada License 98903
In the USA, IBDoc® is available for Investigational Use Only and shall not be used for diagnostic procedures.
IBDoc® and CALEX® are registered trademarks of BUHLMANN Laboratories AG in many countries.





















